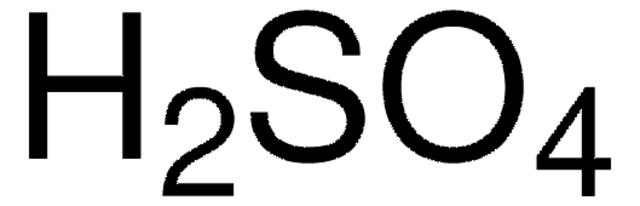Carbon-supported Ni(OH)2 nanospheres decorated with Au nanoparticles: a promising catalyst for BH4− electrooxidation | Ionics
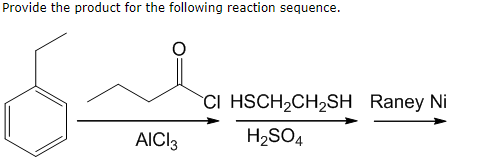
![4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc. 4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc.](https://toppr-doubts-media.s3.amazonaws.com/images/4903703/da785a60-cf16-4f6b-8427-bb073f7a8246.jpg)
4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc.

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink

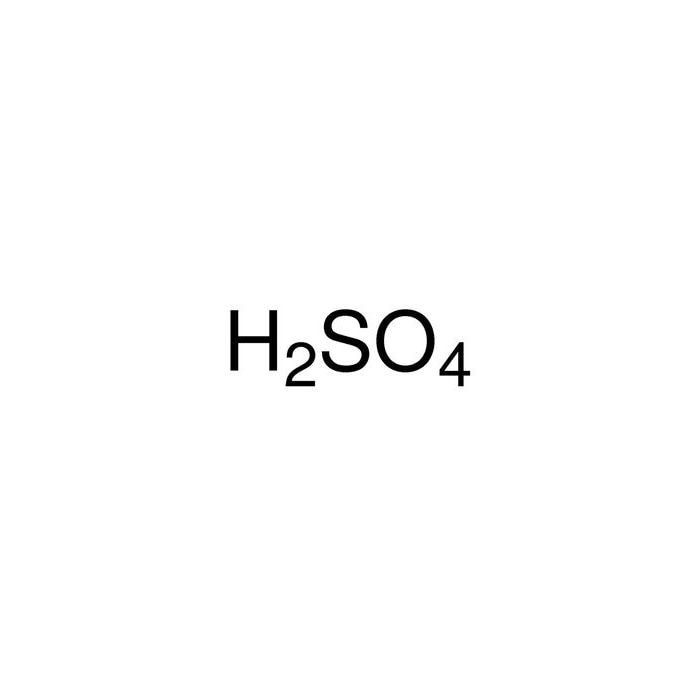
How to balance Ni+H2SO4=Ni2(SO4)3+H2|Chemical equation Ni+H2SO4=Ni2(SO4)3+H2| Ni+H2SO4=Ni2(SO4)3+H2 - YouTube

Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure - ScienceDirect
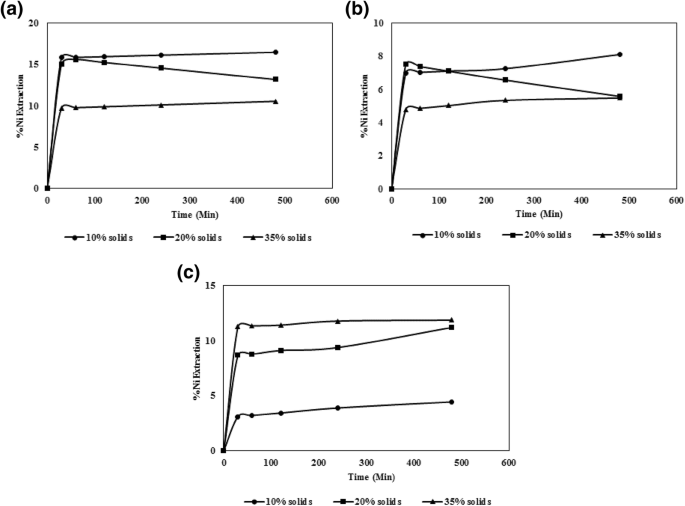
Characteristics of Leaching of Nickel from a Mafic Overburden in Sulfuric Acid and Sodium Chloride Medium at Atmospheric Pressure | SpringerLink